Discover ViBRheo
A multidisciplinary project
• H2020-MSCA-IF-2020 #101021893 •
ViBRheo A virtual diagnostic tool for blood clotting
In coagulation disorders, the body cannot control the process of blood clotting, causing either bleeding or thrombosis. During blood clotting, the viscosity of the blood increases and the flow properties change. The EU-funded ViBRheo project proposes to exploit these alterations in the viscoelasticity of the blood due to SARS-CoV-2 infections for diagnostic purposes. Researchers will develop a computational method that can detect alterations in the microstructural morphology of blood that undergoes clotting. They will also identify biomarkers associated with blood clotting that will assist in non-invasive monitoring of patients suffering from coagulation abnormalities due to SARS-CoV-2.
COVID-19 represents an unprecedented health crisis of this century that has already caused millions of deaths around the world. Significantly, 20 to 30 per cent of critically-ill patients developed coagulopathies, which produce thrombotic complications leading to organ failure and mortality. Currently, a full understanding of the mechanism triggering the clotting remains unclear. The formulation of treatment strategies for critically ill patients is crucial to prevent thrombotic complications and potentially improve survival rates.
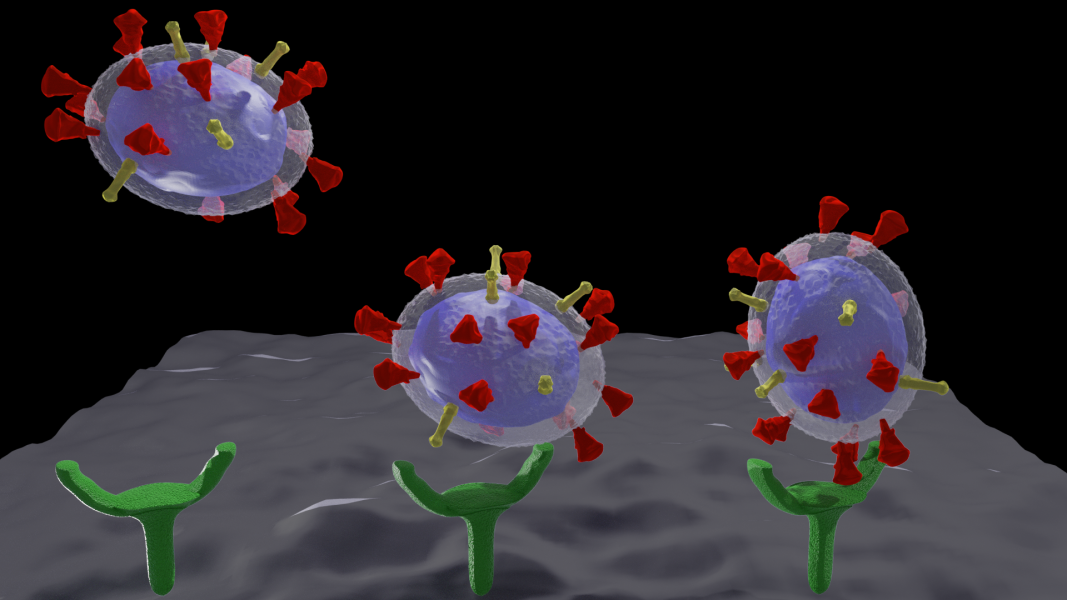
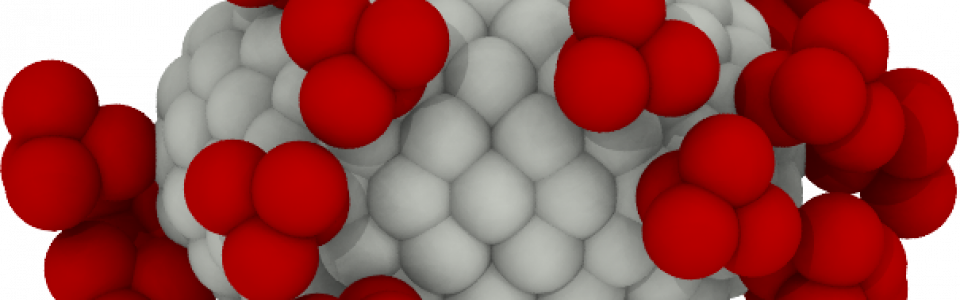
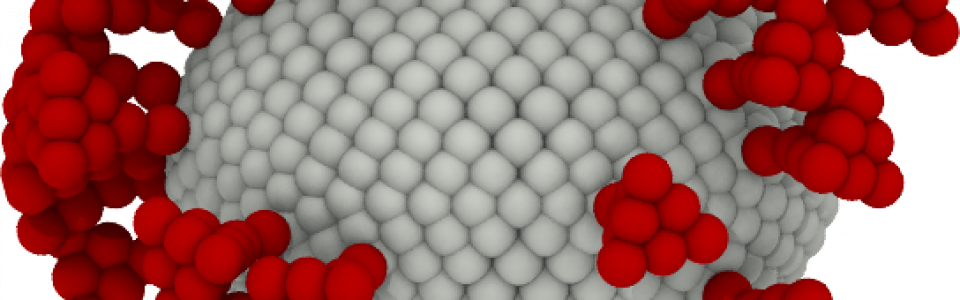
Virus transport
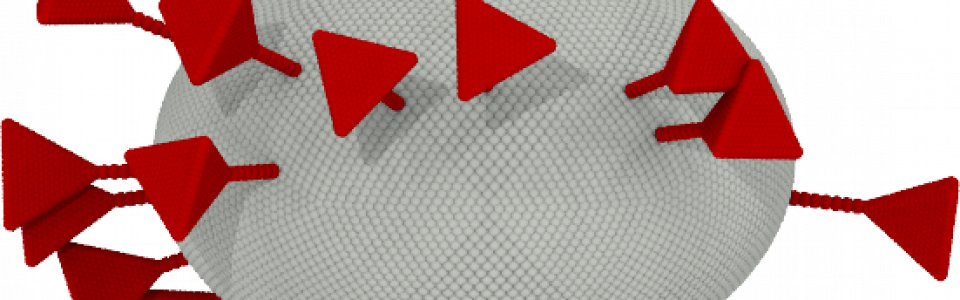
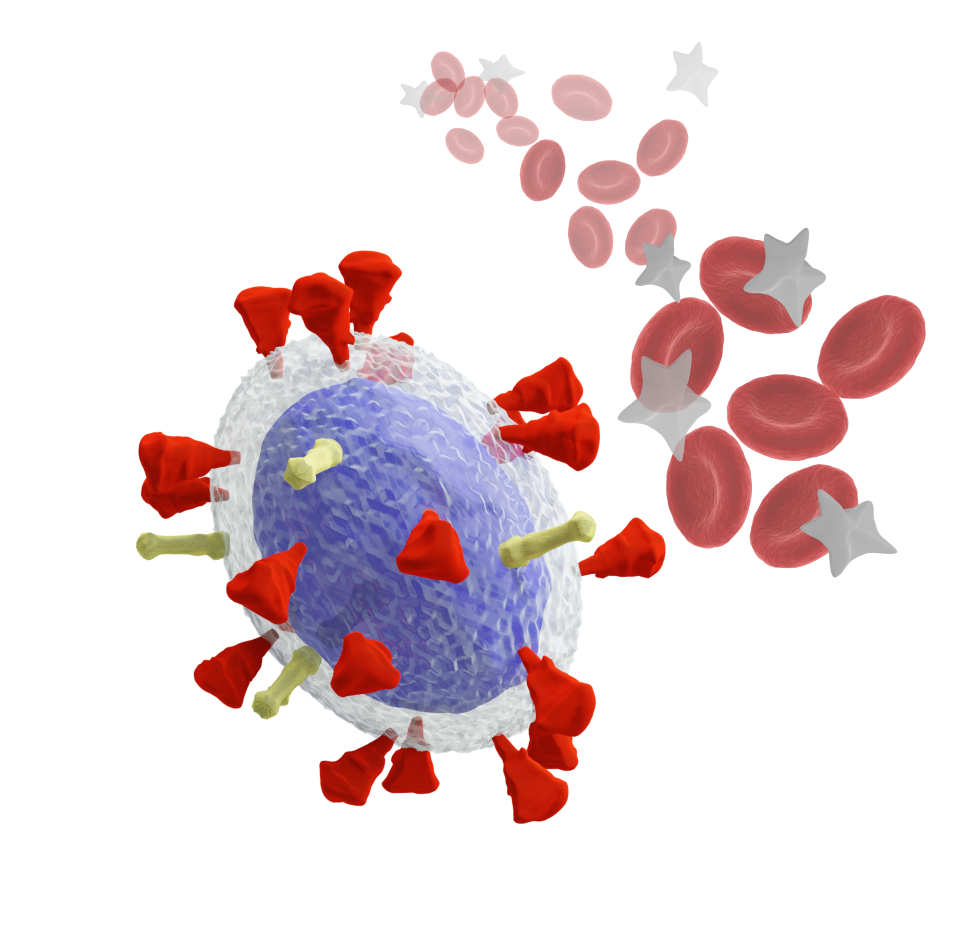
SARS-CoV-2 is responsible for coagulopathies but the mechanisms behind are still under investigation
“Many viruses, such as SARS-CoV-2 or Influenza, possess envelopes decorated with surface proteins (a.k.a. spikes). Depending on the virus type, a large variability is present in the surface proteins number, morphology and reactivity, which remains generally unexplained. Since viruses’ transmissibility depends on features beyond their genetic sequence, new tools are required to discern the effects of spikes’ functionality, interaction, and morphology. Here, we postulate the relevance of hydrodynamic interactions in the viral infectivity of enveloped viruses and propose micro-rheological characterization as a platform for virus differentiation. To understand how the spikes affect virion mobility and infectivity, we investigate the diffusivity of spike-decorated structures using mesoscopic hydrodynamic simulations. Furthermore, we explored the interplay between affinity and passive viral transport. Our results revealed that the diffusional mechanism of SARS-CoV-2 is strongly influenced by the size and distribution of its spikes. We propose and validate a universal mechanism to explain the link between optimal virion structure and maximal infectivity for many virus families”
Moreno, N., Moreno-Chaparro, D., Usabiaga, F.B. et al. Hydrodynamics of spike proteins dictate a transport-affinity competition for SARS-CoV-2 and other enveloped viruses. Sci Rep 12, 11080 (2022). https://doi.org/10.1038/s41598-022-14884-6
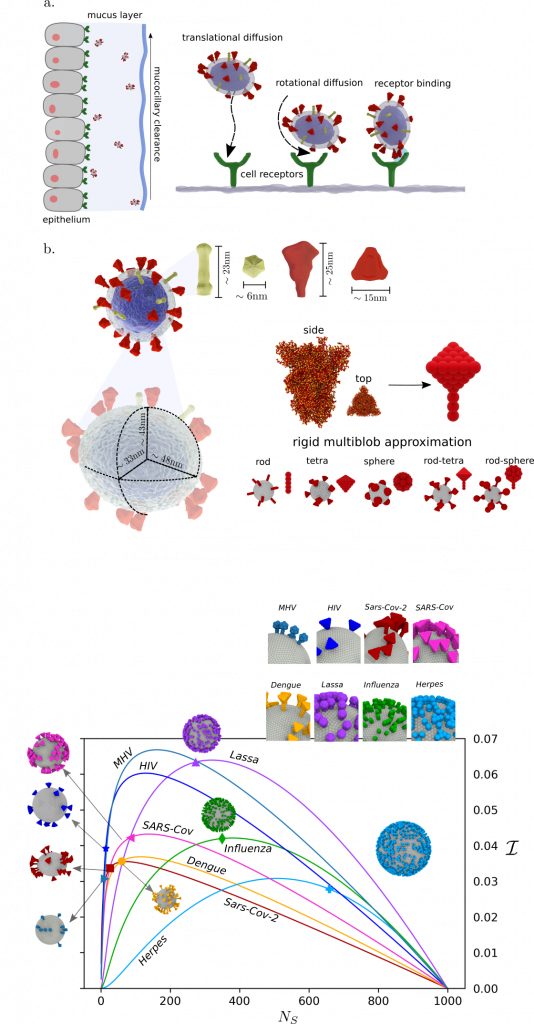
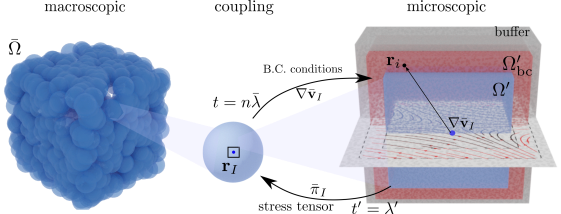
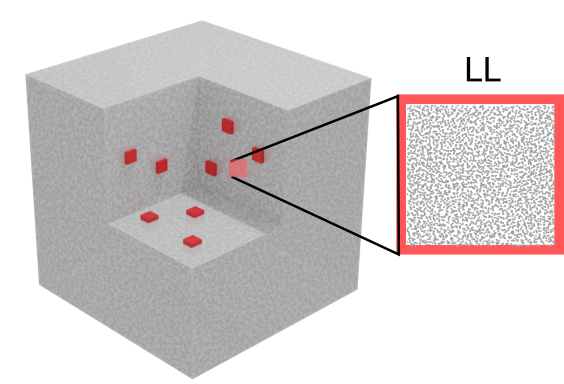
Fully Lagrangian Heterogeneous Multiscale Modelling
Heterogeneous Multiscale Modeling HMM
Heterogeneous multiscale methods (HMM)[Weinan2007] that combine numerical algorithms to resolve separately macro and micro-scales, appear as powerful tools to model the behaviour of fluids across scales. In HMMs, microscales are localized and solved on parts of the domain to obtain microscopically-derived properties that are used to close the macroscale problem[Ren2005]. This methodology offers the advantage of capturing microscopic effects at the macroscopic length scales, with a lower cost than solving the full microscale problem in the whole domain.

Lagrangian HMM
For ViBRheo, we construct a fully-Lagrangian HMM that describes both macro and microscales using a particle-based discretization. This discretization allow us to capture the large relaxation times associated with blood constituents aggregation.
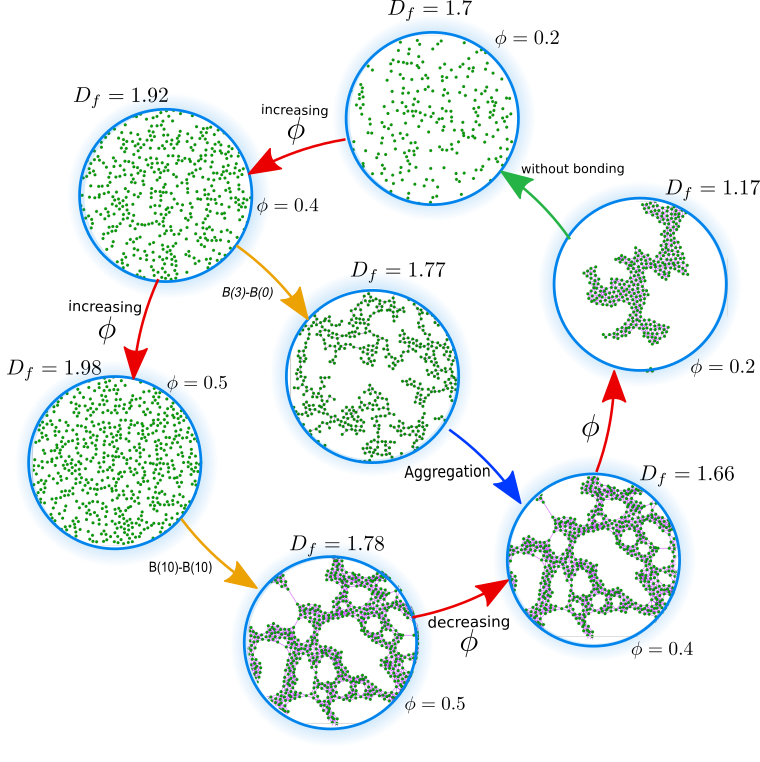
Biological Aggregation Modelling
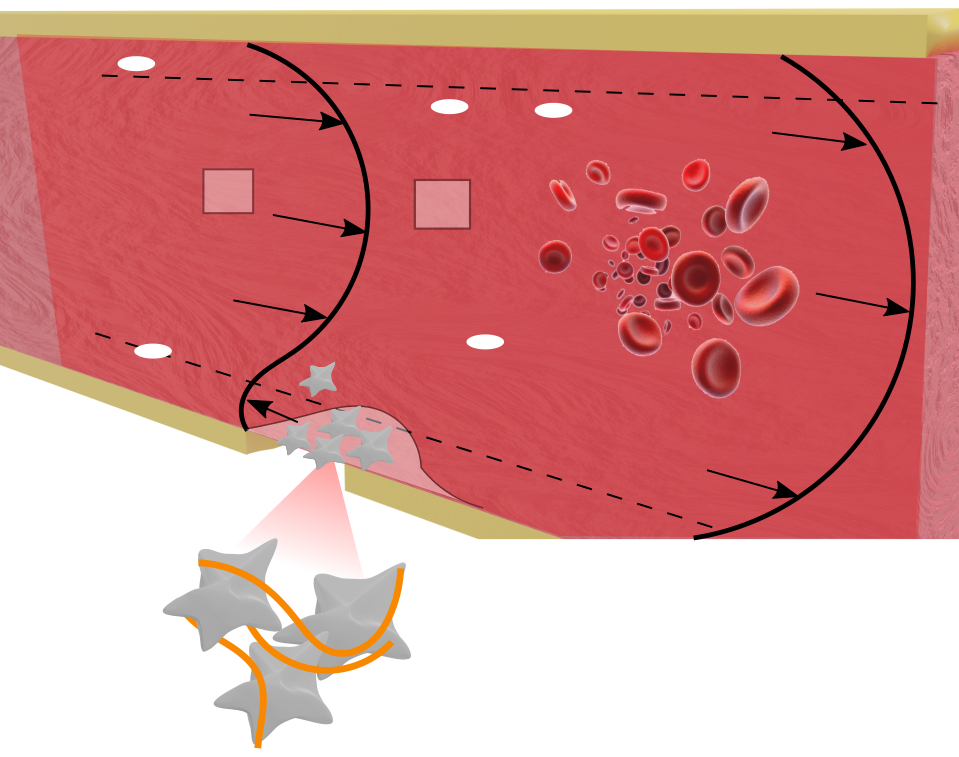
Blood consituents can aggregate in many different ways
Aggregation in blood
In general, different multiscale-aggregation processes occurring during the thrombus formation can be highlighted, including platelet adhesion, aggregation, and growth due to fibrin polymerization. On a cellular length scale, aggregation processes also occur in erythrocytes leading to the formation and settling of microscale disjoint aggregates. From a computational standpoint, the usual approach is to separate the aggregation phenomena in subsystems with narrower length and time scales, focusing on a specific stage of the coagulation cascade. Here, we construct a generlized scheme to investigate biological aggregation at mesoscales. This methodology allow us to characterize aggregates of different nature, depending on the type, constituent, and aggregation stages.